Keith Klugman
Director, Pneumonia, Bill & Melinda Gates Foundation
Every year, 800,000 children die of pneumonia, a well-known but often-neglected disease. Though effective treatment and preventive vaccines exist, pneumonia remains the leading infectious cause of death for children.
Despite the high death toll, bacterial pneumonia – which is particularly serious for kids – only receives about 2% of global funding for neglected disease research and development[1].
Access to diagnostic tools and treatments
like X-rays, antibiotics, or oxygen also remains a challenge, particularly in
low-income countries where most pneumonia deaths occur.
The best option for children in these areas
is to prevent them from getting sick in the first place by giving them the
vaccines they need.
Pneumococcal conjugate vaccines have had major success in high-income countries
One particularly important tool in the
fight against pneumonia are pneumococcal conjugate vaccines (PCV), which have
reduced rates of severe pneumonia by more than half in the high-income
countries that have used them for nearly two decades.
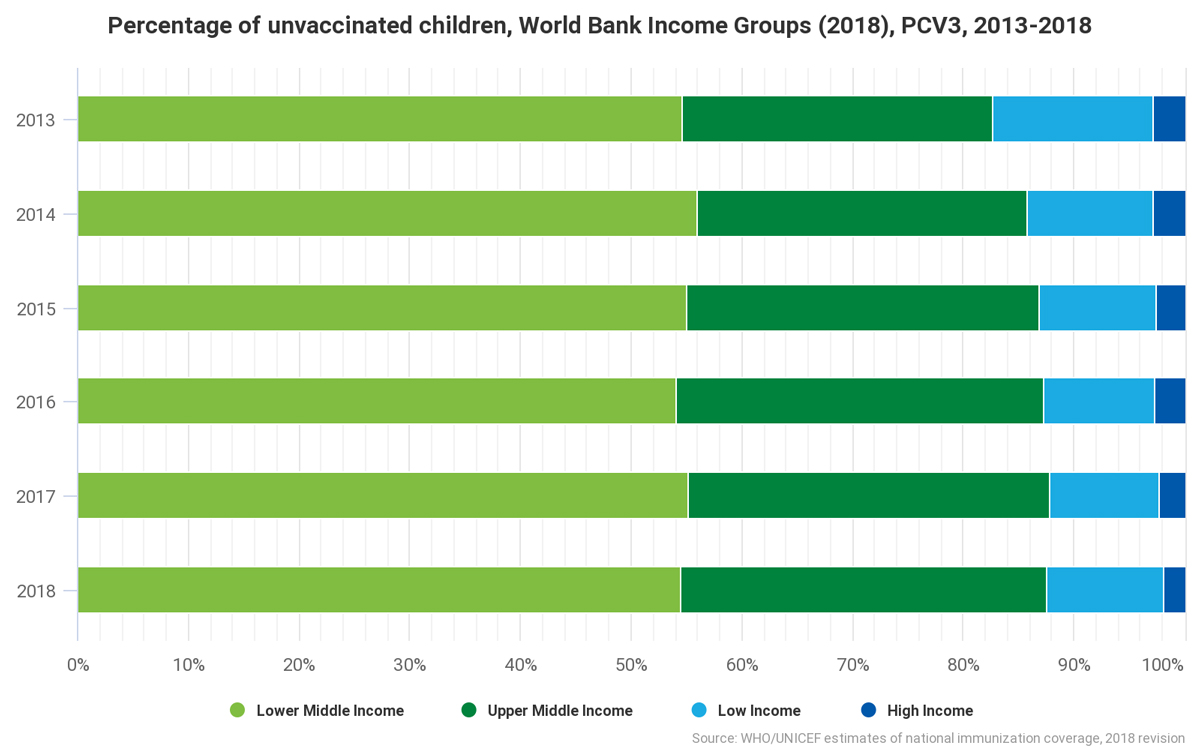
But, while this important tool exists, many
communities in low- and middle-income countries still don’t have access to the
vaccines, leaving millions of children without protection against this deadly
disease.
Thankfully, a new vaccine will soon be on
the market that will help reduce this disparity and make PCVs available to more
children. The availability of this vaccine will help alleviate one of the
biggest barriers to sustainable access to PCVs that countries face – price.
A new
pneumococcal vaccine from the Serum Institute of India was recently approved for
use by the World Health Organization and is expected to be 30%
cheaper for low-income countries than existing vaccines.
Lower-priced vaccines
With the support of organisations like Gavi, the Vaccine Alliance, poor countries
will be better placed than ever before to introduce these vaccines into their
routine immunisation programmes.
Gavi helps increase access to vaccines in low-income countries and has already supported 59 low-income countries to introduce PCVs, reaching more than 183 million children.
With the availability of a more affordable vaccine,
countries will have more options to choose from. The lower price means they can
free up valuable resources for other health or development priorities.
There are encouraging signs of progress. Indonesia
announced in January that it
would make PCV part of its routine immunisation programme and committed to
vaccinating four million children each year. Rolling PCVs out in a country like
Indonesia, with a large population and a high burden of pneumonia, is a major step
forward.
Pneumonia prevention must be a priority
Reducing deaths from pneumonia in the
long-term will require putting pneumonia at the top of the global agenda and
keeping it there.
High-burden countries must make protecting
children from pneumonia through well-functioning primary healthcare systems a top
priority.
Donor governments must continue to
generously fund organisations like Gavi to ensure countries have the support they
need to introduce PCVs and sustain their use in every community.
To create a world free of preventable
disease, we must ensure every child can access these life-saving vaccines – no
matter where they live.
[1] (Policy Cures Research. G-FINDER 2019: Neglected Disease Research and Development: Uneven Progress, Jan 2020.)